Syntheses, crystal structures, surface photovoltage, luminescence and molecular recognition properties of zinc(ii) and iron(ii) carboxyphosphonates with 2D and 3D supramolecular structures†
Abstract
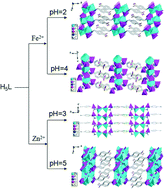
Four new transition metal carboxyphosphonates with 2D and 3D supramolecular structures, namely, Fe2[(HL)(H2O)] (1), Fe(H4L)2 (2), Zn(H3L) (3) and Zn2(HL) (4) (H5L = 4-HO2C–C6H4–CH2N(CH2PO3H2)2), have been synthesized under hydrothermal conditions. For compound 1, {FeO5N} and {CPO3} polyhedra are interconnected into a 2D layer in the bc-plane via corner-sharing. Then the adjacent layers are further assembled into a 3D supramolecular structure through π–π stacking interactions. Compound 2 shows a 3D supramolecular structure. {FeO6} and {CPO3} polyhedra are interconnected into a 2D layer in the bc-plane via corner-sharing, which is further linked through π–π stacking interactions to form a 3D supramolecular structure. The overall structure of compound 3 can be described as a 2D supramolecular structure. The {ZnO4} polyhedra are interconnected by {CPO3} tetrahedra via corner-sharing to form a 1D chain. These neighboring metal phosphonate chains are connected through hydrogen bonding interactions to give rise to a 2D supramolecular structure in the ac-plane. In compound 4, {ZnO3N} and {ZnO4} polyhedra are interconnected by {CPO3} tetrahedra via corner-sharing and edge-sharing to form a 2D inorganic layer in the bc-plane, which is further linked through π–π stacking interactions to form a 3D supramolecular structure. The surface photovoltage properties of compounds 1–2 and luminescence properties of compounds 3–4 have been investigated. More interestingly, compound 4 is selective for sensing DMF and acetone.

 Please wait while we load your content...
Please wait while we load your content...