Synthesis, structures and properties of the first series of SrII–MII (M = Cu, Co, Ni and Zn) coordination polymers based on pyridine-2,5-dicarboxylic acid†
Abstract
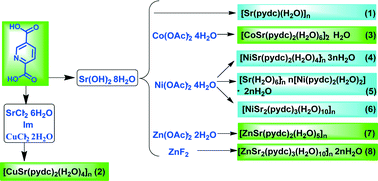
The first series of heterometallic SrII–MII (M = Cu, Co, Ni and Zn) coordination polymers based on pyridine-2,5-dicarboxylic acid (H2pydc) are reported. Eight complexes of compositions, [Sr(pydc)(H2O)]n (1), [CuSr(pydc)2(H2O)4]n (2), [CoSr(pydc)2(H2O)6]2n·H2O (3), [NiSr(pydc)2(H2O)4]n·3nH2O (4), [Sr(H2O)6]n·n[Ni(pydc)2(H2O)2]·2nH2O (5), [NiSr2(pydc)3(H2O)8]n·2nH2O (6), [ZnSr(pydc)2(H2O)5]n (7) and [ZnSr2(pydc)3(H2O)10]n·2nH2O (8), have been prepared and characterized. The structures of the complexes could be controlled via rationally choosing the appropriate metal salts, adding the starting materials in different orders, and tuning the ratio of the starting materials. Eight varieties of structures, ranging from 1-D chains to 3-D networks, were obtained. The magnetic properties of 2, 3 and 6 were investigated from 2 K to 300 K. Antiferromagnetic MII⋯MII interactions were found for 2 and 6, and ferromagnetic interactions were determined for 3. The solid state luminescent properties of 1, 7 and 8 were also studied.

 Please wait while we load your content...
Please wait while we load your content...