Hydrogen-bond networks in polymorphs and solvates of metallorganic complexes containing ruthenium and aminoamide ligands†
Abstract
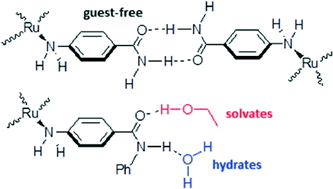
Half-sandwich ruthenium(II) complexes containing the two amino-amide ligands 4-aminobenzamide (4AB) and 4-aminobenzanilide (4ABN) have been synthesized and structurally characterized. The complexes have the general formula {[(p-cymene)RuCl2(κN-ligand)](x·solvent)}. The crystal packings are governed by extended hydrogen-bond networks involving the N–H bonds of the amine and amide groups as well as the Cl ligands. In the case of 4AB, two different non-solvate polymorphs (x = 0) have been isolated from methanol (1α) and water (1β, disappearing polymorph). In both cases, the amide groups of two molecules associate, giving rise to a supramolecular ring, where the N–H bond not involved in the ring intermolecularly contacts a neighbouring Cl ligand. The resultant frameworks are very robust, thus impeding the insertion of solvent molecules. A methanol-solvate (1·MeOH) has in fact been isolated in very low yield where two methanols bridge two different amide groups. These crystals are highly unstable at room temperature. However, 1α shows a distinct reactivity toward NH3 in heterogeneous gas-uptake experiments, although the final nature of the product has not yet been defined. A comparison between the structural results reported here and those previously collected with 4-aminobenzoic acid reveals that the COOH function leads to linear supramolecular wheel-and-axle motifs, while the C(O)NH2 function of 4AB leads to bent supramolecular wheel-and-axle motifs. The complexes containing 4ABN (2 and 2·2H2O) have always been crystallized as solvate (2·EtOH and 2·H2O), where the included guest molecules are hydrogen-bonded to the C(O)NHR functionality, thus preventing the dimerization of the amide groups.

 Please wait while we load your content...
Please wait while we load your content...