Silver nanowires with rounded ends: ammonium carbonate-mediated polyol synthesis, shape evolution and growth mechanism
Abstract
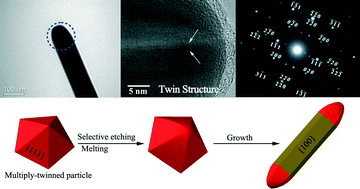
Under atmospheric conditions, silver nanowires were prepared via an (NH4)2CO3-mediated polyol process rather than the normally used metal salt-mediated polyol processes in order to improve the purity. The results revealed that the obtained silver nanowires had rounded ends rather than the conventionally believed {111} crystal planes. A growth mechanism was firstly proposed, in which a dynamic reversible deposition and dissolution process of Ag atoms occurred at the curved ends of the silver nanowires. The proposed mechanism could explain the effects of the titration rate, PVP and (NH4)2CO3 concentrations on the growth the of silver nanowires. Both melting and dissolution of Ag atoms contributed to the arcing of twin boundaries in multiply-twinned particles. The multiply-twinned particles with arcing twin boundaries finally grew into silver nanowires with rounded end shapes and higher aspect ratios. Lattice fringes of the twin structure were observed for the first time at the curved ends of the silver nanowires, and the addition of (NH4)2CO3 was found to facilitate the growth of the silver nanowires.

 Please wait while we load your content...
Please wait while we load your content...