Copper(ii) and cobalt(ii) complexes based on bis-benzimidazolyl ligand with 1,2-bis(2′-ethoxy)phenyl linker: synthesis, crystal structure and conformations†
Abstract
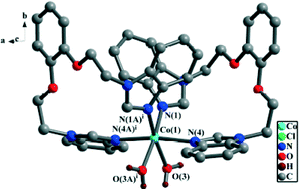
Seven Cu(II) and Co(II) complexes [Co(L)2(H2O)2]Cl2 (1), [Cu(L)Cl2]2 (2), [Cu(L)(NO3)2]2 (3), [Cu(L)(SO4)]2 (4), [Co(L)(LA)]n (5), [Cu(L)(OAc)2] (6) and [Co(L)Cl2] (7) (L = 1,2-bis(2′-ethoxy)phenyl-bis(benzimidazole), LA = terephthalate) have been prepared by means of self-assembly of Cu(II) or Co(II) salts with bis-benzimidazolyl chelate ligand L and a 1,2-bis(2′-ethoxy)phenyl linker. These complexes are structurally characterized by X-ray diffraction analyses. In complexes 1–4, two 15-membered macrometallocycles of each molecule are linked together via sharing moieties (the sharing moieties: Co(II) atom for 1, two bridging chlorine atoms for 2, two bridging nitrate groups for 3 and two bridging sulfate groups for 4) to afford a dimer, in which each 15-membered macrometallocycle is constructed from one ligand L and one metal atom (Co(II) for 1 and Cu(II) for 2–4). In complex 5, a 1D polymeric chain with 15-membered macrometallocycles is formed via ligand L, terephthalate and a Co(II) atom. In complexes 6 and 7, each molecule contains a 15-membered macrometallocycle formed by one ligand L and one metal atom (Cu(II) for 6 and Co(II) for 7). In the crystal packings of 1–7, 2D supramolecular layers or 3D supramolecular frameworks are formed via intermolecular weak interactions, including hydrogen bonds, π–π interactions and C–H⋯π contacts. The conformations of the metal complexes based on ligand L are described. Additionally, the fluorescence emission spectra of ligand L and the metal complexes 1–7, and the magnetic properties for complexes 1–7 are reported.

 Please wait while we load your content...
Please wait while we load your content...