Development of a redox-free Mitsunobu reaction exploiting phosphine oxides as precursors to dioxyphosphoranes†
Abstract
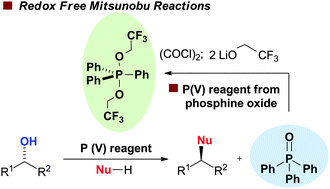
The development of the first redox-free protocol for the Mitsunobu reaction is described. This has been achieved by exploiting triphenylphosphine oxide – the unwanted by-product in the conventional Mitsunobu reaction – as the precursor to the active P(V) coupling reagent. Multinuclear NMR studies are consistent with hydroxyl activation via an alkoxyphosphonium salt.

 Please wait while we load your content...
Please wait while we load your content...