Synthesis of the 6,6,5,7-tetracyclic core of daphnilongeranin B†
Abstract
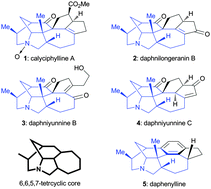
An efficient approach toward the synthesis of the 6,6,5,7-tetracyclic core of the daphnilongeranin B, a Daphniphyllum alkaloid, is reported. The bridged 6,6-bicyclic system was constructed using a gold(I) catalysed Conia-ene reaction, while the 5- and 7-membered rings were assembled by two diastereoselective Michael addition reactions, respectively.
- This article is part of the themed collections: Celebrating the ChemComm Emerging Investigator Lectureship Winners and 2014 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...