A novel chimeric amine dehydrogenase shows altered substrate specificity compared to its parent enzymes†
Abstract
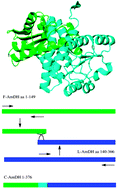
We created a novel chimeric amine dehydrogenase (AmDH) via domain shuffling of two parent AmDHs (‘L- and F-AmDH’), which in turn had been generated from leucine and phenylalanine DH, respectively. Unlike the parent proteins, the chimeric AmDH (‘cFL-AmDH’) catalyzes the amination of acetophenone to (R)-methylbenzylamine and adamantylmethylketone to adamantylethylamine.

 Please wait while we load your content...
Please wait while we load your content...