A seco-catechin cyclization approach to 4→6-linked catechin dimers†
Abstract
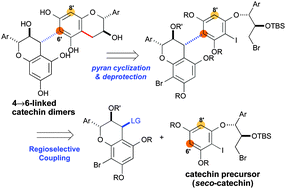
A viable route has been developed for the selective synthesis of the 4→6-linked catechin dimers, scarcely accessible from Nature and/or through synthesis. An acyclic nucleophilic catechin precursor (seco-catechin) was used for the regioselective union with an electrophilic catechin unit, and subsequent pyran cyclization gave the desired 4→6-linked dimers, i.e., procyanidin B6 and catechin-(4α→6)-gallocatechin.

 Please wait while we load your content...
Please wait while we load your content...