Chemically-induced redox switching of a metalloprotein reveals thermodynamic and kinetic heterogeneity, one molecule at a time†
Abstract
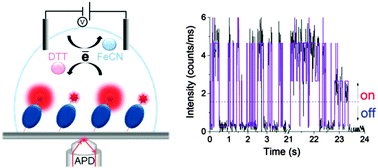
Oxidation (off state) and reduction (on state) of a single azurin molecule is monitored, one electron at a time, which depend on the chemical redox potential. By analysing the fluorescence time traces from individual azurin molecules, reaction kinetics and redox thermodynamics were determined.

 Please wait while we load your content...
Please wait while we load your content...