Enantioselective protonation of α-hetero carboxylic acid-derived ketene disilyl acetals under chiral ionic Brønsted acid catalysis†
Abstract
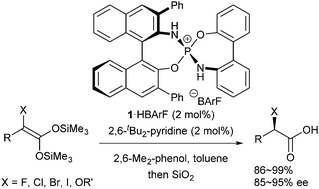
Highly enantioselective protonation of α-halo and alkoxy carboxylic acid-derived ketene disilyl acetals is achieved by using P-spiro chiral diaminodioxaphosphonium barfate as a Brønsted acid catalyst, where the enantiofacial discrimination by the catalyst mainly stems from the recognition of the electronic difference between two substituents on the ketene disilyl acetal.

 Please wait while we load your content...
Please wait while we load your content...