Substituent controlled reactivity switch: selective synthesis of α-diazoalkylphosphonates or vinylphosphonates via nucleophilic substitution of alkyl bromides with Bestmann–Ohira reagent†
Abstract
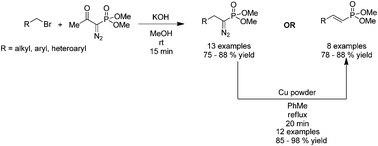
We report a substituent controlled nucleophilic displacement of alkyl bromides with Bestmann–Ohira reagent yielding either dimethyl diazoalkylphosphonates or (E)-vinylphosphonates. The dimethyl diazoalkylphosphonates could be readily converted into corresponding (E)-vinylphosphonates in the presence of Cu following nitrogen elimination in quantitative yields.

 Please wait while we load your content...
Please wait while we load your content...