Fluorometric assay of integrin activity with a small-molecular probe that senses the binding site microenvironment†
Abstract
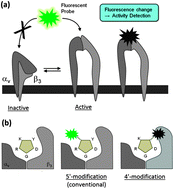
We have developed a small-molecular probe, consisting of cyclic RGD pentapeptides bearing a nitrobenzoxadiazole fluorophore at the 4′-residue, which detects integrin αVβ3 activity in terms of fluorescence intensity decrease due to quenching of the fluorophore by interaction with tyrosine-122 at the binding site of the protein. This probe appears to be suitable for a practical, high-throughput fluorescence assay to screen small-molecular modulators of integrin activity.

 Please wait while we load your content...
Please wait while we load your content...