H2Me-do2pa: an attractive chelator with fast, stable and inert natBi3+ and 213Bi3+ complexation for potential α-radioimmunotherapy applications†
Abstract
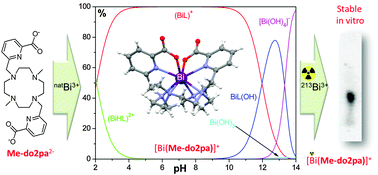
The complexation properties of H2Me-do2pa towards natBi3+ reveal a rather fast formation of the [Bi(Me-do2pa)]+ complex, which is endowed with a very high thermodynamic stability (log KBiL = 34.2) and presents a single non-fluxional structure in solution. X-ray diffraction and solution NMR studies showed an octadentate binding of the ligand to the metal ion. The labelling of H2Me-do2pa with 213Bi was performed and the resulting complex was stable in vitro, sustaining its use as an attractive alternative to H4dota taken here as a reference.

 Please wait while we load your content...
Please wait while we load your content...