Cu-Catalyzed Suzuki–Miyaura reactions of primary and secondary benzyl halides with arylboronates†
Abstract
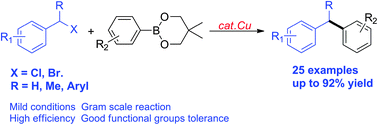
A copper-catalyzed Suzuki–Miyaura coupling of benzyl halides with arylboronates is described. Varieties of primary benzyl halides as well as more challenging secondary benzyl halides with β hydrogens or steric hindrance could be successfully converted into the corresponding products. Thus it provides access to diarylmethanes, diarylethanes and triarylmethanes.

 Please wait while we load your content...
Please wait while we load your content...