Consecutive oxygen-based oxidations convert amines to α-cyanoepoxides†
Abstract
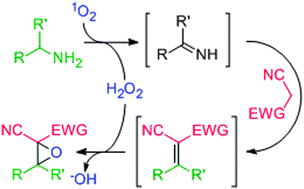
Tri- or tetrasubstituted α-cyanoepoxides can be rapidly prepared from unactivated amines and malononitrile or methyl cyanoacetate when singlet oxygen, produced in a continuous-flow photoreactor, serves as an oxidant and in situ peroxide source. The hydrogen peroxide generated in amine oxidation epoxidizes an electron deficient olefin intermediate, formed by deaminative Mannich coupling. The corresponding α,α-dicyano- or α-cyano-α-esterepoxides were obtained in good yields (43–82%).


 Please wait while we load your content...
Please wait while we load your content...