Synthesis of 3,3-disubstituted indoline-2-thiones catalysed by an N-heterocyclic carbene†
Abstract
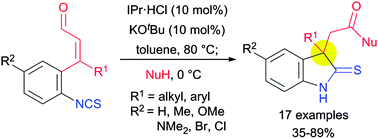
A catalytic method has been developed for construction of indoline-2-thiones containing an all-carbon quaternary centre at the C-3 position. Successive treatment of α,β-unsaturated aldehydes bearing an isothiocyanato moiety with an in situ generated N-heterocyclic carbene and an appropriate heteroatomic nucleophile provided the 3,3-disubstituted indoline derivatives in moderate to good yields.

 Please wait while we load your content...
Please wait while we load your content...