Polygermanes: bandgap engineering via tensile strain and side-chain substitution†
Abstract
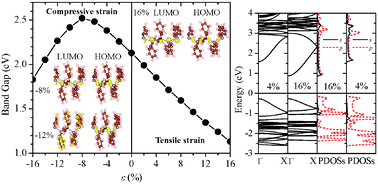
Successful synthesis of the phenylisopropyl hexagermane (Chem. Commun. 2013, 49, 8380) offers an exciting opportunity to synthesize a new class of low-dimensional germanium compounds with novel optical and electronic properties. Using the phenylisopropyl hexagermane as a model template, we have performed an ab initio study of electronic properties of polygermanes. Our density functional theory calculations show that the polygermane is a quasi-one-dimensional semiconductor with a direct bandgap, and its valence and conduction bands are mainly contributed by the skeletal Ge atoms. We have also explored effects of tensile and compressive strains and various side-chain substituents on the bandgap. The bandgap of polygermanes can be reduced upon attaching larger-sized substituents to the side chains. More importantly, applying a tensile/compressive strain can modify the bandgap of polygermanes over a wide range. For poly(diphenlygermane), the tensile strain can result in significant bandgap reduction due to the increasingly delocalized charge density in the conduction band. Moreover, a strong compressive strain can induce a direct-to-indirect semiconductor transition owing to the change made in the band-edge states. A similar strain effect is seen in polystannanes as well.

 Please wait while we load your content...
Please wait while we load your content...