Meso enamine substituted BODIPYs†
Abstract
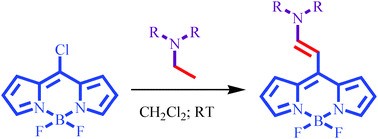
Different enamines were introduced at the meso position of the BODIPY by catalyst free oxidation of tert-amines and in situ cross coupling with 8-chloro BODIPY. The reaction conditions were optimized to achieve better yields. The reaction works well with aliphatic tert-amines bearing an N-(CH–CH–) backbone. The N-alkyl substituents perturb the optical properties of enamine substituted BODIPYs.

 Please wait while we load your content...
Please wait while we load your content...