A general metal free approach to α-ketoamides via oxidative amidation–diketonization of terminal alkynes†
Abstract
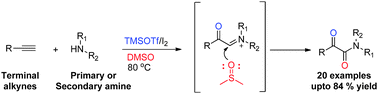
A novel catalytic system TMSOTf/I2/DMSO for the oxidative coupling of terminal alkynes with virtually any primary/secondary amine leading to α-ketoamides has been developed. The reaction possibly proceeds via iminium ion formation, wherein DMSO acts as a solvent as well as an oxidizing agent.

 Please wait while we load your content...
Please wait while we load your content...