A kinetically protected pyrene: molecular design, bright blue emission in the crystalline state and aromaticity relocation in its dicationic species†
Abstract
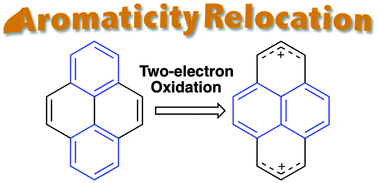
Sterically congested tetraarylpyrenes exhibited emission in both solution and the solid state. The monocationic species of pyrene 1 could be isolated because of the reasonably protected system. The aromaticity of 1 relocates from the biphenyl part to the naphthalene unit upon two-electron oxidation.

 Please wait while we load your content...
Please wait while we load your content...