Abstract
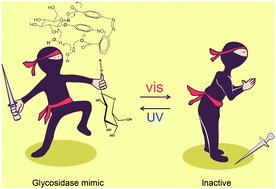
Azobenzene-3,3′-dicarboxylic acid exists in photoisomerizable (E) and (Z)-forms. Deprotonation of the carboxylic acid groups from the (E)-form occurs simultaneously, whereas in the (Z)-form it occurs in a stepwise fashion. The mono anionic form of the (Z)-isomer acts as a glycosidase mimic that proceeds through a general acid–general base catalytic mechanism. This is the first example of a photoresponsive glycosidase mimic.

 Please wait while we load your content...
Please wait while we load your content...