Facile synthesis of fluorescent active triazapentalenes through gold-catalyzed triazole–alkyne cyclization†
Abstract
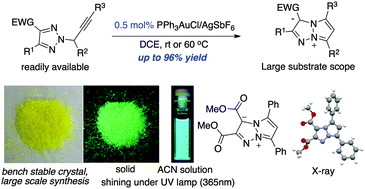
Fluorescent active triazapentalene zwitterions (TAPZs) were prepared through Au(I) catalyzed triazole–alkyne 5-endo-dig cyclization. While an effective gold catalyst turnover (0.5% loading, up to 96% yield) was achieved, the stability of these new 10-π-electron bicyclic structures was also significantly improved, which warranted future applications of these fluorescent dyes.

 Please wait while we load your content...
Please wait while we load your content...