Activation of a carbonyl compound by halogen bonding†
Abstract
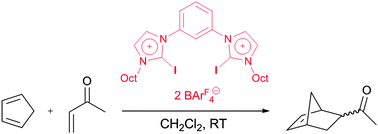
Using a prototypical Diels–Alder reaction as benchmark, we show that dicationic halogen-bond donors are capable of activating a neutral organic substrate. By various comparison experiments, the action of traces of acid or of other structural features of the halogen-bond donor not related to halogen bonding are excluded with high certainty.

 Please wait while we load your content...
Please wait while we load your content...