Biomass derived furfural-based facile synthesis of protected (2S)-phenyl-3-piperidone, a common intermediate for many drugs†
Abstract
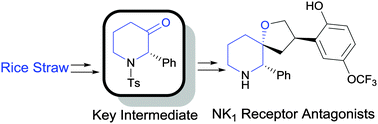
An efficient synthetic route towards tosyl-protected (2S)-phenyl-3-piperidone, a common intermediate for many drugs, has been developed in 5 steps in 54% yield from biomass derived furfural. The synthetic utility of the piperidone core structure was demonstrated with the synthesis of a NK1 receptor antagonist.

 Please wait while we load your content...
Please wait while we load your content...