Dihydrothiophenes containing quaternary stereogenic centres by sequential stereospecific rearrangements and ring-closing metathesis†‡
Abstract
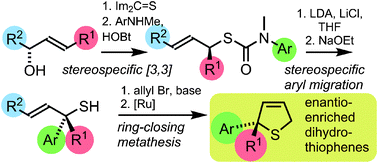
Stereospecific [3,3]-sigmatropic rearrangement of O-substituted thiocarbamate derivatives of enantiopure allylic alcohols provides allylic thiocarbamates as single enantiomers. Intramolecular arylation by rearrangement of their allyllithium derivatives provides allylic tertiary thiols. Allylation and ring-closing metathesis gives 2,5-dihydrothiophenes containing sulfur-bearing quaternary centres.
- This article is part of the themed collection: In Celebration of Richard Taylor’s 65th Birthday

 Please wait while we load your content...
Please wait while we load your content...