Enantioselective synthesis of benzazepinoindoles bearing trifluoromethylated quaternary stereocenters catalyzed by chiral spirocyclic phosphoric acids†
Abstract
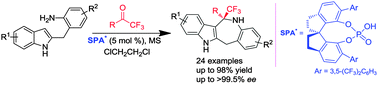
The first highly enantioselective iso-Pictet–Spengler reaction of C-2-linked o-aminobenzylindoles with trifluoromethyl ketones was developed using chiral spirocyclic phosphoric acids as organocatalysts, which afforded optically active benzazepinoindoles bearing trifluoromethylated quaternary stereocenters.

 Please wait while we load your content...
Please wait while we load your content...