Asymmetric synthesis of functionalized cyclohexanes bearing five stereocenters via a one-pot organocatalytic Michael–Michael–1,2-addition sequence†
Abstract
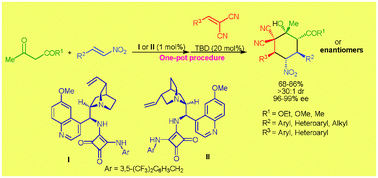
A highly stereoselective one-pot procedure involving an enantioselective Michael addition promoted by low loading of an amino-squaramide catalyst followed by an achiral base catalyzed domino Michael–Knoevenagel-type 1,2-addition sequence provides efficient access to fully substituted cyclohexanes bearing five contiguous stereogenic centers in good yields (68–86%) and excellent stereoselectivities (>30 : 1 dr and 96–99% ee).

 Please wait while we load your content...
Please wait while we load your content...