Effects of heteroatom substitution in conjugated heterocyclic compounds on photovoltaic performance: from sulfur to tellurium†
Abstract
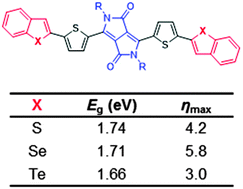
We report a general strategy for fine-tuning the bandgap of donor–acceptor–donor based organic molecules by modulating the electron-donating ability of the donor moiety by changing the benzochalcogenophene donor groups from benzothiophenes to benzoselenophenes to benzotellurophenes. These molecules show red-shifts in absorption and external quantum efficiency maxima from sulfur to selenium to tellurium. In bulk heterojunction solar cell devices, the benzoselenophene derivative shows a power conversion efficiency as high as 5.8% with PC61BM as the electron acceptor.

 Please wait while we load your content...
Please wait while we load your content...