A heavy metal- and oxidant-free, one-pot synthesis of pyridines and fused pyridines based on a Lewis acid-catalyzed multicomponent reaction†
Abstract
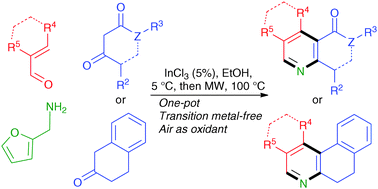
The InCl3-catalyzed sequential multicomponent reaction between 2-furfurylamine, β-dicarbonyl compounds and α,β-unsaturated aldehydes in ethanol, followed by microwave irradiation in solvent-free conditions, afforded good to excellent yields of highly substituted pyridines, with loss of a 2-furylmethyl side chain. The method was also adapted to the synthesis of quinolones, isoquinolines, phenanthridines and more complex fused pyridine systems.

 Please wait while we load your content...
Please wait while we load your content...