On the hydrates of codeine phosphate: the remarkable influence of hydrogen bonding on the crystal size†
Abstract
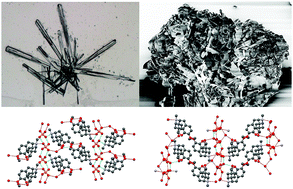
Codeine phosphate forms three hydrates and two anhydrates. The sesquihydrate and hemihydrate, which differ by one water molecule, are stable at room temperature. The influence of this molecule on the internal crystal structure and how it translates into the external crystal shape are reported.

 Please wait while we load your content...
Please wait while we load your content...