Dimensionality changes in the solid phase at room temperature: 2D → 1D → 3D evolution induced by ammonia sorption–desorption on zinc phosphates†
Abstract
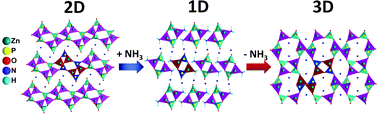
Two-dimensional zinc phosphate NH4Zn2(PO4)(HPO4) (1), via ammonia vapor interaction at room temperature, transforms to a one-dimensional novel compound NH4Zn(NH3)PO4 (2). By ammonia desorption (in air at room temperature) 2 transforms to NH4ZnPO4 (3) with a well-known ABW-zeolitic topology. The crystal structure of 2 was solved ab initio using synchrotron powder X-ray diffraction data (monoclinic, P21/a, a = 16.5227(2) Å, b = 6.21780(8) Å, c = 5.24317(6) Å, β = 91.000(2)°, Z = 4). The structures of three compounds include extra-framework ammonium cations to the 4-fold coordinated zinc (ZnO4 tetrahedra for 1 and 3, and ZnO3N tetrahedra for 2) and phosphorus (PO4 tetrahedra) with bi-, mono- or three-dimensional linkages, respectively for 1, 2 or 3. To our knowledge, the process described here constitutes the first example of dimensionality change in the solid phase promoted by a solid–gas interaction at room temperature in metal phosphates.

 Please wait while we load your content...
Please wait while we load your content...