Li–Se battery: absence of lithium polyselenides in carbonate based electrolyte†
Abstract
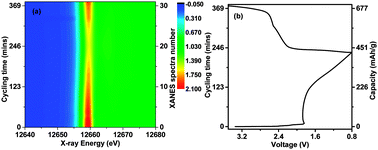
The lithiation mechanism of the Li–Se cell in a carbonate-based electrolyte is discussed. It is found that Se is directly reduced to Li2Se in discharge without intermediate phases detected by in situ X-ray diffraction or X-ray absorption spectroscopy. The reason is that the redox products Se and Li2Se, as well as lithium polyselenides are insoluble in the electrolyte.

 Please wait while we load your content...
Please wait while we load your content...