Oxidative coupling of methylamine with an aminyl radical: direct amidation catalyzed by I2/TBHP with HCl†
Abstract
Oxidative coupling of methylamines with an aminyl radical to construct amides was developed in the presence of an I2/TBHP catalyst under acidic conditions via the two cleavages of the sp3 C–N bond of aryl-methylamines and the sp2 C–N bond of N-substituted formamides respectively. This transition-metal-free protocol provides a novel synthetic tool for the construction of N-substituted amides and a series of arylamides can be easily obtained with good yields.
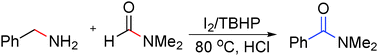

 Please wait while we load your content...
Please wait while we load your content...