β-Hydroxy-γ-lactones as nucleophiles in the Nicholas reaction for the synthesis of oxepene rings. Enantioselective formal synthesis of (−)-isolaurepinnacin and (+)-rogioloxepane A†
Abstract
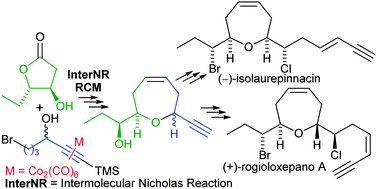
The enantioselective formal synthesis of (−)-isolaurepinnacin and (+)-rogioloxepane A has been achieved. The key steps are an intermolecular Nicholas reaction with a β-hydroxy-γ-lactone as the nucleophile, to form branched linear ethers, and an olefin ring-closing metathesis to obtain the oxepene core.

 Please wait while we load your content...
Please wait while we load your content...