Palladium-catalyzed aerobic oxidative coupling of enantioenriched primary allylic amines with sulfonyl hydrazides leading to optically active allylic sulfones†
Abstract
A range of highly enantioenriched primary allylic amines underwent palladium-catalyzed oxidative coupling with sulfonyl hydrazides open to air at room temperature to give structurally diverse allylic sulfones in moderate to excellent yields with excellent retention of ee.
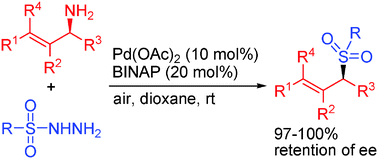

 Please wait while we load your content...
Please wait while we load your content...