Halocarbocyclization versus dihalogenation: substituent directed iodine(iii) catalyzed halogenations†
Abstract
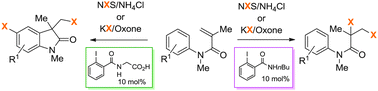
The nucleophilicity of the substituents in iodobenzene pre-catalysts have a huge impact on product selectivity in iodine(III) triggered halogenations, steering the reactivity from solely carbocyclizations towards dihalogenations. Utilizing this catalyst-dependent reactivity a diastereo- and chemoselective dihalogenation method was established allowing the conversion of structurally and electronically diverse unsaturated compounds in excellent yields.

 Please wait while we load your content...
Please wait while we load your content...