Catalytic enantioselective intramolecular cyclization of N-aryl diazoamides using a titanium–BINOLate complex†
Abstract
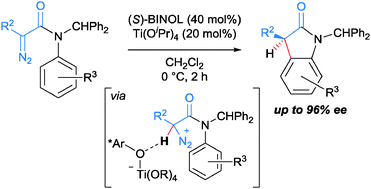
Described herein is the use of chiral titanium(IV)–BINOLate to formally implement catalytic asymmetric C(sp2)–H insertion using N-aryl α-diazoamides. The reaction most likely proceeds via initial asymmetric protonation at the α-carbon of the substrate, followed by the intramolecular electrophilic aromatic substitution.
- This article is part of the themed collection: J400: Celebrating the 400th year of Japan-UK relations

 Please wait while we load your content...
Please wait while we load your content...