Ruthenium porphyrin catalyzed diimination of indoles with aryl azides as the nitrene source†
Abstract
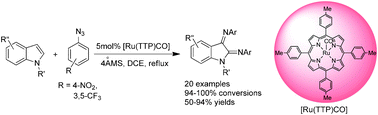
By using [Ru(TTP)CO] [H2TTP = meso-tetrakis(4-tolyl)porphyrin] as catalyst and aryl azides as the nitrene source, the sp2(C–H) bonds of a series of indoles undergo oxidative C–N bond formation to give unique 2,3-diimination products in good to high yields.

 Please wait while we load your content...
Please wait while we load your content...