Observations on transition metal free biaryl coupling: potassium tert-butoxide alone promotes the reaction without diamine or phenanthroline catalysts†
Abstract
Biaryl coupling (often labelled ‘C–H activation’) of aromatic systems can be achieved by potassium tert-butoxide alone in the absence of any amine or bipyridine catalyst (1,10-phenanthroline or N,N′-dimethylethylenediamine being the most common), previously reported to be essential. Various mechanistic studies and observations are presented which suggest that when 1,10-phenanthroline is employed as the catalyst, the alkoxide is destroyed almost immediately.
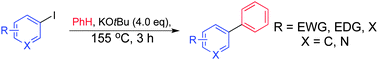

 Please wait while we load your content...
Please wait while we load your content...