En route to multicatalysis: kinetic resolution of trans-cycloalkane-1,2-diols via oxidative esterification†
Abstract
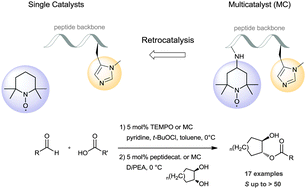
We demonstrate the application of a multicatalyst to the oxidation of a broad variety of aldehydes and subsequent enantioselective esterification of the incipient acids with (±)-trans-cycloalkane-1,2-diols. This reaction operates well with a multicatalyst bearing two independent catalytic moieties that provide monoprotected 1,2-diols in one pot.

 Please wait while we load your content...
Please wait while we load your content...