Regiodivergent heterocyclization: a strategy for the synthesis of substituted pyrroles and furans using α-formyl ketene dithioacetals as common precursors†
Abstract
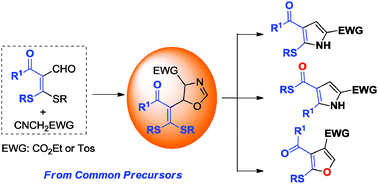
The selective construction of a diverse array of five-membered aromatic heterocycles from common starting materials is an attractive and challenging task. We report here that 5-alkylthio-pyrroles, 4-alkylthiocarbonyl-pyrroles and 2-alkylthio-4-tosyl-furans can be prepared, selectively, in a single operation using the readily available α-formyl ketene dithioacetals as common precursors, through their regiodivergent heterocyclization with α-acidic isocyanides controlled by the choice of suitable catalysts/promoters.

 Please wait while we load your content...
Please wait while we load your content...