Gold catalysed synthesis of 3-alkoxyfurans at room temperature†
Abstract
Synthetically important 3-alkoxyfurans can be prepared efficiently via treatment of acetal-containing propargylic alcohols (obtained from the addition of 3,3-diethoxypropyne to aldehydes) with 2 mol% gold catalyst in an alcohol solvent at room temperature. The resulting furans show useful reactivity in a variety of subsequent transformations.
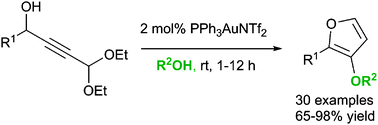

 Please wait while we load your content...
Please wait while we load your content...