Total synthesis of sulfolipid-1†
Abstract
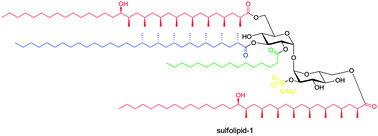
Sulfolipid-1, a tetra-acylated sulfotrehalose from Mycobacterium tuberculosis, was isolated over 40 years ago. Being a main component of the mycomembrane of M. tuberculosis, its biosynthesis and function have been studied in depth, but the chemical synthesis of sulfolipid-1 has not been reported. The synthesis presented here is based on iterative catalytic asymmetric conjugate additions of methylmagnesium bromide for the preparation of the phthioceranic and hydroxyphthioceranic acid side chains, a double regioselective reductive ring-opening and a fivefold deprotection in the final step.

 Please wait while we load your content...
Please wait while we load your content...