Pd-catalyzed allylic alkylation of dienyl carbonates with nitromethane with high C-5 regioselectivity†
Abstract
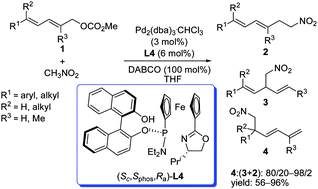
A highly regioselective palladium-catalyzed allylic alkylation of dienyl esters with nitromethane has been developed, providing selective access to the C-5 attacked products. The structures of the ligands as well as the steric effect of the substrates are important factors in determining the regiochemical outcome of the reaction.

 Please wait while we load your content...
Please wait while we load your content...