Conversion of nitrite to nitric oxide at zinc via S-nitrosothiols†
Abstract
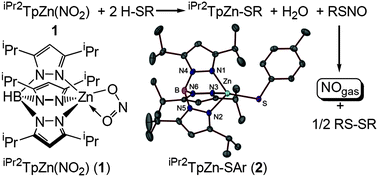
Nitrite is an important reservoir of nitric oxide activity in the plasma and cells. Using a biomimetic model, we demonstrate the conversion of zinc-bound nitrite in the tris(pyrazolyl)borate complex iPr2TpZn(NO2) to the corresponding S-nitrosothiol RSNO and zinc thiolate iPr2TpZn–SR via reaction with thiols H–SR. Decomposition of the S-nitrosothiol formed releases nitric oxide gas.

 Please wait while we load your content...
Please wait while we load your content...