Reversible photochromism of an N-salicylidene aniline anion†
Abstract
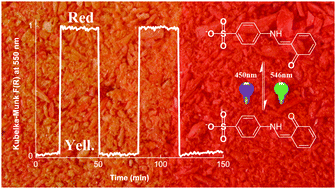
The first N-salicylidene aniline anion showing reversible solid state thermochromic and photochromic properties is described. The photo-isomerization involves a trans-keto form which is stabilized thanks to the local anion surrounding. This photochromic anion can be used as a guest for the preparation of hybrid materials by insertion into a cationic host matrix.

 Please wait while we load your content...
Please wait while we load your content...