Peptide decoration of nanovehicles to achieve active targeting and pathology-responsive cellular uptake for bone metastasis chemotherapy†
Abstract
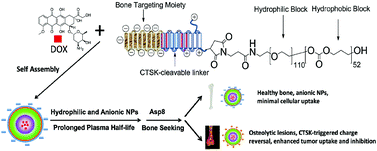
To improve bone metastasis chemotherapy, a peptide-conjugated diblock copolymer consisting of a chimeric peptide, poly(ethylene glycol) and poly(trimethylene carbonate) (Pep-b-PEG-b-PTMC) is fabricated as a drug carrier capable of bone-seeking as well as pathology-responsive charge reversal to ensure effective cellular uptake at the lesion sites. The chimeric peptide CKGHPGGPQAsp8 consists of an osteotropic anionic Asp8, a cathepsin K (CTSK)-cleavable substrate (HPGGPQ) and a cationic residue tethered to the polymer chain. Pep-b-PEG-b-PTMC can spontaneously self-assemble into negatively charged nanomicelles (∼75 nm). As to the model drug of doxorubicin, Pep-b-PEG-b-PTM shows 30.0 ± 1% and 90.1 ± 2% for the loading content and loading efficiency, respectively. High bone binding capability is demonstrated with 66% of Pep-b-PEG-b-PTMC micelles were able to bind to hydroxyl apatite, whereas less than 15% of Pep-free micelles were bound to hydroxyl apatite. The nanomicelles exhibit a negative-to-positive charge conversion from −18.5 ± 1.9 mV to 15.2 ± 1.8 mV upon exposure to CTSK, an enzyme overexpressed in bone metastatic microenvironments. Such a pathology-responsive transition would lead to remarkably enhanced cellular uptake of the nanomicelles upon reaching lesion sites, thus improving the drug efficacy as verified by the in vitro cytotoxicity assay and the in vivo study with the myeloma-bearing 5TGM1 mice model.
 Please wait while we load your content...
Please wait while we load your content...