Electrochemical study and differential pulse voltammetric determination of oxcarbazepine and its main metabolite at a glassy carbon electrode
Abstract
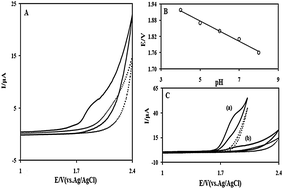
In this paper, the electrochemical behavior of oxcarbazepine (OX) and its main metabolite monohydroxy carbamazepine (MHD) was studied by cyclic voltammetry (CV) at a glassy carbon electrode. The effect of factors such as type of supporting electrolytes and solvent, potential scan rates and pH on the voltammetric behaviour of OX and MHD was investigated by CV. The results indicated one irreversible anodic peak at the potential Epa = 1. 84 V for OX and two irreversible anodic peaks at potentials EA1 = +1. 64 V and EA2 = +1.84 V for MHD. The oxidation reactions for both compounds (OX and MHD) were shown to be diffusion controlled. A possible mechanism is proposed to explain the electrochemical oxidation of OX. A differential pulse voltammetric method was used for the determination of OX in tablet and MHD in plasma samples. Based on this study, the calibration plots to determine OX and MHD concentrations were linear in the range of 2–23 μM and 0.35–18 μM, respectively. The detection limits were found to be 0.81 μM for OX and 0.135 μM for MHD. The results indicate that the proposed method is sensitive, selective, fast and simple for determination of OX and MHD.

 Please wait while we load your content...
Please wait while we load your content...