The selective interaction between N-isobutyryl-cysteine enantiomers and l-methotrexate
Abstract
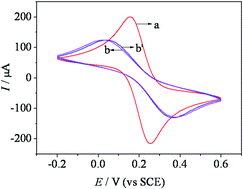
Simple and sensitive chiral interfaces were constructed by assembling L- or D-N-isobutyryl-cysteine (NIBC) on a gold electrode surface through an Au–S bond, and the two chiral interfaces were utilized to interact with L-methotrexate (L-Mtx). The surface characteristics and the process of interaction were explored via cyclic voltammetry (CV). The difference in peak current after L- and D-NIBC interacted with L-Mtx reached 525.3 μA, and a larger current difference was obtained from the L-NIBC interface, suggesting a stereoselective effect between L-Mtx and NIBC enantiomers. Therefore, the chiral NIBC interfaces could not only enhance the electron transfer of L-Mtx, but could also discriminate between NIBC enantiomers.

 Please wait while we load your content...
Please wait while we load your content...